HAE - Hereditary Angioedema
PATHOGENESIS & CLINIC
Hereditary Angioedema (HAE) is a disorder caused by a quantitative or functional defect of the C1 inhibitor (C1-INH). In rare cases, the defect is acquired (Acquired Angioedema, AAE). The C1-INH defect leads to increased vascular permeability via activation of the kinin system, which occurs in the following clinical manifestations:
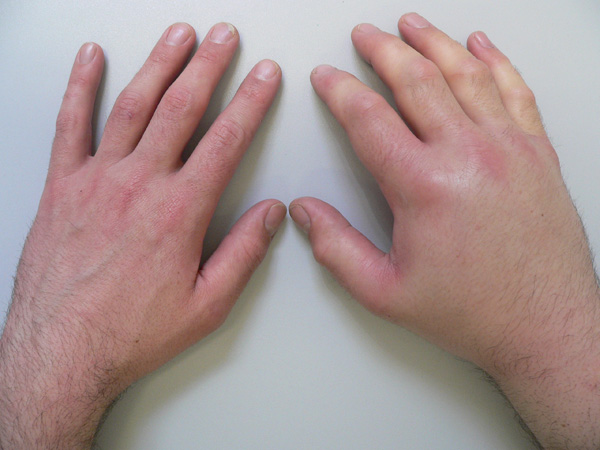
1) Cutaneous angioedema (frequent):
There is ongoing angioedema of the skin and subcutaneous tissue for days. The extremities, face and genitalia are most often affected.
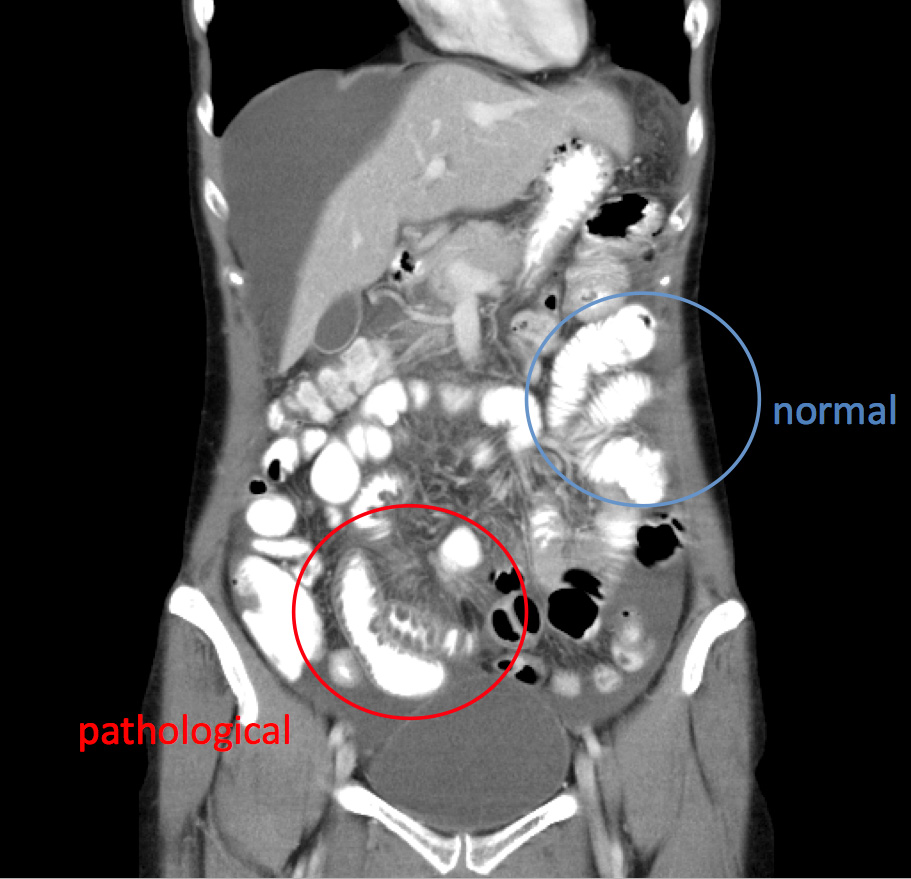
2) Abdominal attacks (frequent):
Attacks in the gastrointestinal area are associated with ongoing colicky pain, nausea, often associated with vomiting, diarrhoea and circulatory dysregulation for days. Imaging procedures often show ascites and/or swelling of the intestinal loops.
3) Laryngeal edema:
Mucosal swelling in the area of the larynx can rapidly develop into complete obstruction of the airway and cause death. Early symptoms are dysphagia, dysphonia and stridor. The uvula and tongue may also be affected and cause dyspnea.
4) Other localisations:
Edema at other locations (brain, muscles, bladder, etc.) is rare, symptoms depend on the severity of the edema and location.
TREATMENT
Angioedema of the head and neck or other internal organs is a medical emergency and must be treated immediately. For laryngeal edema, the indication for intensive care must be aggressive, as intubation or even coniotomy may become necessary. Cutaneous angioedema should be treated if it is painful, functionally impairing or progressive. The treatment of acute angioedema caused by a C1-INH defect is:
1) C1-INH concentrate (Berinert P):
Dose: Adults and children, 20 units per kg of body weight.
Application: Carefully dissolve 500 units in 10 ml of the included solvent and slowly administer intravenously.
Berinert P consists of two components: a vial containing protein concentrate (500 U human C1 inhibitor) and a vial of solvent (10 ml of water for injection). The pack additionally contains all the medical devices which are required for preparing the solution for injection and for intravenous administration of Berinert P. The storage temperature of the preparation must not exceed 25°C.
If the preparation has been stored in a refrigerator, both components should be adjusted to room temperature prior to preparation. The solution for injection is prepared using the enclosed needleless Mix2VialTM filter transfer set (follow instructions for use). Once the solvent has been transferred into the product vial, the protein quickly dissolves. To avoid foaming, do not shake the product vial. The protein solution should be clear to slightly opalescent and must not contain any clearly visible particles. Any waste should be properly disposed of after use.
or
2) C1-INH concentrate (Cinryze)
Dose: Adults and children, 1000 units.
Application: Carefully dissolve 500 units in 5 ml of the included solvent and slowly administer intravenously.
Cinryze consists of two components: a vial containing protein concentrate (500 U human C1 inhibitor) and a vial of solvent (5 ml of water for injection). The pack additionally contains all the medical devices which are required for preparing the solution for injection and for intravenous administration of Cinryze. The storage temperature of the preparation must not exceed 8°C.
Both components should be adjusted to room temperature prior to preparation. The solution for injection is prepared using the enclosed transfer set (follow instructions for use). Once the solvent has been transferred into the product vial, the protein quickly dissolves. To avoid foaming, do not shake the product vial. The protein solution should be colorless to slightly blue, should be clear, and must not contain any clearly visible particles. Any waste should be properly disposed of after use.
or
3) C1-INH concentrate (Ruconest)
Dose: Adults, 50 units per kilogram of body weight up to a maximum dose of 4200 units.
Application: Carefully dissolve 2100 units in 14 ml of water for injection and slowly administer intravenously.
Ruconest consists of a vial containing protein concentrate (2100 U recombinant C1 inhibitorderived from rabbit milk). Hypersensitivity to rabbits must be excluded before application (specific IgE anti-rabbit). The storage temperature of the preparation must not exceed 25°C.
The solution for injection is prepared using 14ml of water for injection per vial by following the instructions for use. Once the solvent has been transferred into the product vial, the protein quickly dissolves. To avoid foaming, do not shake the product vial. The protein solution should be colorless and clear, and must not contain any clearly visible particles. The quantity to be administered in relation to body weight is calculated according to the following formula:
Quantity to be administered in ml = (Body weight in kg) / 3
The prepared solution is slowly administered intravenously. Any waste should be properly disposed of after use.
or
4) Icatibant (Firazyr)
Dose: Adults, 30 mg Icatibant in 3 ml solution as ready-to-use syringe
Application: Inject s.c. slowly and deeply
Antihistamines, corticosteroids, danazol or tranexamic acid are not effective or ineffective for an acute attack.
MONITORING
The initial effects of C1-INH concentrate or Icatibant appear after approx. 20-40 minutes. For known or threatening obstruction of the airway, (intensive care) inpatient monitoring until complete remission. For abdominal attacks or edema of the skin, monitoring until clear clinical improvement has occurred. Follow-up care may be necessary.